
Your Regulatory and CRO Partner in IVD & Medical Devices
From early development to post-market success, MDx delivers precision-driven regulatory and clinical solutions tailored to the unique needs of MedTech innovators.
Company Overview
MDx CRO is a specialist Contract Research Organization (CRO) and regulatory consultancy for the in vitro diagnostics (IVD) and medical device sectors. With offices in Barcelona, London, Madrid, and Lisbon and regulatory experts across Europe, US and APAC MDx supports companies navigating the complex global frameworks of IVDR, MDR, ISO standards, MHRA, CE-marking, FDA and clinical trials.
Founded by leaders with decades of experience in notified bodies, regulatory operations, and clinical research, MDx combines precision strategy with deep regulatory insight — delivering expert support from first concept to post-market success.

Our Mission and Vision
Mission
Vision
What We Do
Regulatory Affairs
Clinical Research (CRO Services)
Quality & Compliance
Regulatory Representation
Training
Our Values
We solve complex regulatory challenges with practical, expert solutions.
We believe in shared success through strong, collaborative partnerships.
Continuous learning and improvement is core to our service and our people.
We scale with our clients — from startup to market leader.
Diverse teams drive better decisions, innovation, and outcomes.
Carlos Galamba – CEO
Former IVD global technical manager and clinical lead at a BSI, a leading notified body and an advisor to the EU Commission on regulatory matters. Deep expertise in Class C/D IVDs, companion diagnostics, and clinical evidence generation.

David Tome – President
Specialist in global clinical operations and regulatory strategy. Extensive experience in medical devices, diagnostics, and SaMD. Known for supporting MedTech innovators through scale-up and trial design.

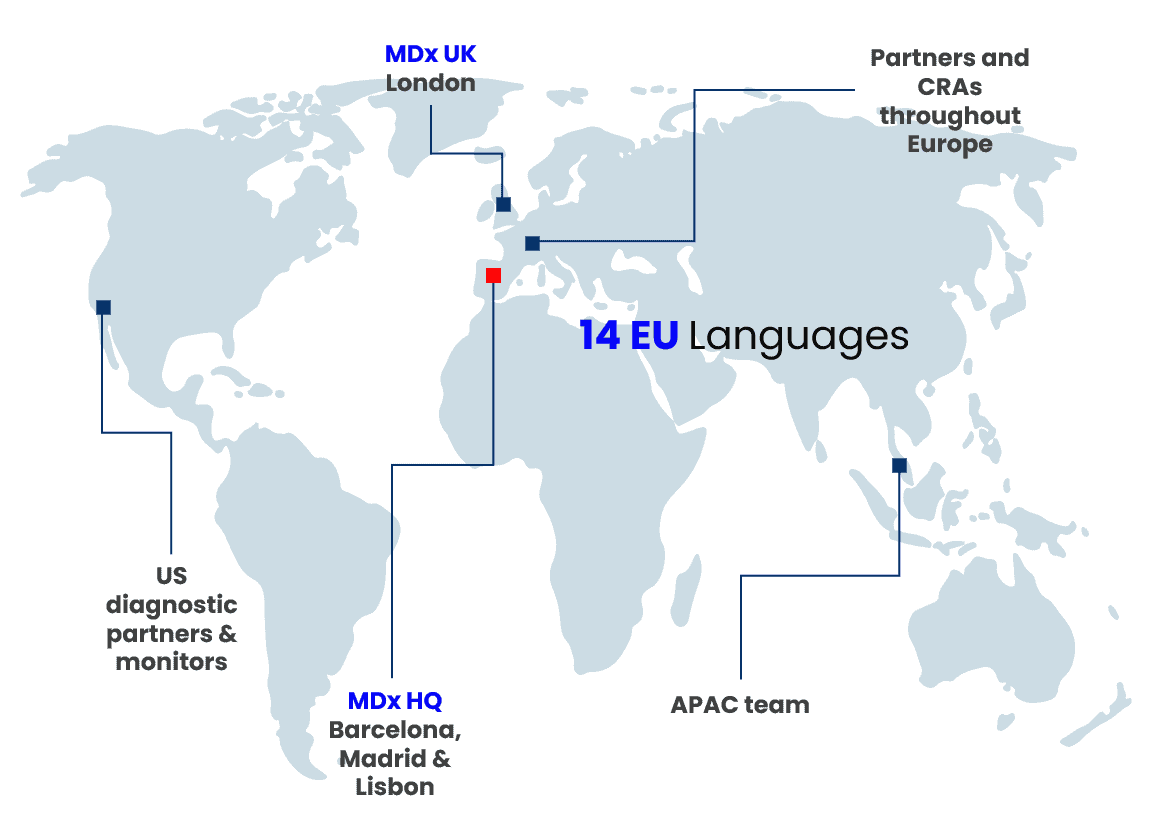
- Offices in Barcelona, Madrid, Lisbon & London
- Clinical & regulatory partners across the EU, UK, US and APAC
- Collaborators with Notified Bodies, Trade Associations, Medtech & Pharma Platform (MPP) and more
- Fluent in 14 EU languages
- Representation across key global markets
| Feature |
|---|
| Dual Regulatory + CRO Expertise |
| IVD & Device Focus |
| Representation + Execution |
| Global Regulatory Reach |
| Agile and Scalable |
| Notified Body & Authority Liaison |
| MDx CRO |
|---|

Work with a partner who speaks your regulatory language.
MDx combines expert regulatory insight with clinical excellence — helping MedTech teams succeed at every stage.
Precision. Partnership. Performance.

