
Your Precision Partner for Companion Diagnostics & Biomarker-Driven Trials
MDx CRO is a precision medicine and companion diagnostics CRO helping IVD and pharma teams design and run CDx clinical studies. We manage CDx development from biomarker validation through IVDR compliance, FDA Q-submissions, and ISO 20916-compliant clinical execution.
Full-Service CDx CRO for Precision Medicine
MDx CRO is a specialist companion diagnostics CRO helping pharma and IVD teams plan and run companion diagnostics clinical studies. We align biomarker strategy and assay validation with IVDR Annex XIV and FDA PMA/Q-submission pathways to accelerate market access in Europe (EU/UK) and the US.
From SRD vs NSRD pathway selection and Q-sub preparation to ISO 20916 study design and monitoring, our team delivers end-to-end CDx CRO execution—including sponsor delegation and EU/UK legal representative services.

Your Companion Diagnostic Journey
Fully Managed, Step by Step
ONE GOAL
We guide precision medicine sponsors through each critical phase, aligning clinical, regulatory, and operational workflows to accelerate your path to approval.
Define your development pathway with expert guidance on FDA and IVDR requirements — including SRD vs NSRD determinations, Q-sub meetings, and PMA submissions. Our regulatory intelligence spans the US & EU.
Support for biomarker discovery, clinical trial assay development, analytical validation, and translational biomarker strategy. Includes assay validation for qPCR, NGS, IHC, and SaMD platforms.
We qualify lab vendors, LDTs, and test sites under ISO 15189 and CE mark requirements, conducting due diligence for test suitability and compliance with IVDR for clinical trial assays destined for CDx claims.
From CPSP and endpoint definition to risk management and informed consent, we design and run ISO 20916-compliant clinical trials. MDx can act as your EU/UK legal representative.
End-to-end clinical operations — site qualification, training, monitoring, and audit readiness — ensuring protocol adherence and GCP alignment with ISO 20916 on-site and remote monitoring.
We manage eCRF design, statistical analysis, and development of clinical performance study reports for IVDR submissions, including clinical performance study reports for IVDR Annex XIV.
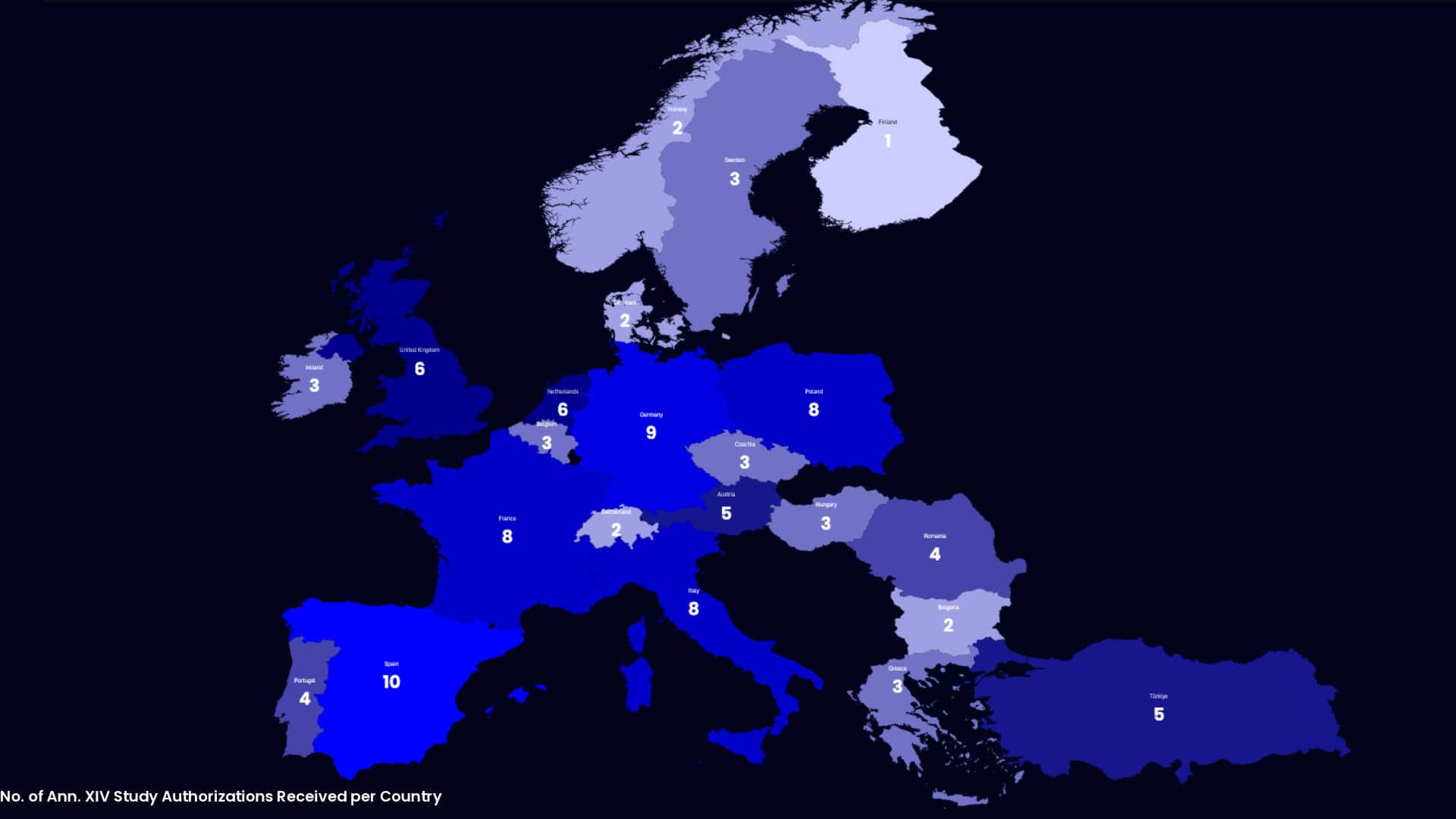
From FDA PMA & Q-submission packages; IVDR Annex XIV applications, we handle all ethics and competent authority communications, RFIs, and modifications.
Adverse event classification and response, including Notified Body and Ethics Committee communications. Covers IVDR safety reporting in clinical trials.
| Feature or Capability |
|---|
| IVDR Annex XIV Submissions Support (CDx) |
| CDx Consulting (FDA + EU) |
| ISO 20916 Monitoring (CDx) |
| Sponsor Delegation & EU/UK Legal Rep |
| IVD Documentation Development |
| Translational & Biomarker Expertise |
| Regulatory Intelligence |
| MDx CRO | Other CROs | Internal Teams |
|---|---|---|
Therapeutic Areas & Technologies
Therapeutic Focus
Technologies & Platforms
Biomarker Applications

Who We Serve
- Global Pharma co-developing companion diagnostics
- IVD & CDx manufacturers
- Oncology-focused diagnostics developers
- US-based sponsors running companion diagnostics clinical studies in Europe
- Digital diagnostic and software platforms

Commercial CDx Programs Delivered
MDx has successfully supported performance study execution and regulatory submissions for commercialized CDx programs worldwide.
We support companion diagnostics from clinical validation through FDA PMA/Q-submission and IVDR Annex XIV applications, aligning CDx timelines with drug development, including EMA and Notified Body consultations.
Our track record includes:
- HER2 IHC assays for breast and gastric cancer
- EGFR mutation detection kits for NSCLC
- NGS panels with CDx claims (CRC and solid tumors)
- qPCR & multiplex assays validated under IVDR and FDA
Frequently Asked Questions
Ask us a Question-
What is the SRD vs NSRD decision for CDx in the US?
The FDA distinguishes Significant Risk Device (SRD) from Non-Significant Risk Device (NSRD) studies. We guide CDx sponsors through Q-sub interactions and determine the right path toward PMA or other submissions.
-
What is IVDR Annex XIV for companion diagnostics?
Annex XIV defines the clinical performance study requirements for IVDs, including CDx, in the EU. It covers submissions, ethics/competent authority processes, and evidence expectations for IVDR conformity assessment.
-
Can MDx act as legal representative for CDx and IVDR performance studies in the EU?
Yes. We offer delegated sponsor services, ethics/competent authority submissions, and legal representation — managing documentation and communications on your behalf.
-
Do you support both IVDR and FDA PMA or Q-submissions for CDx?
Absolutely. We define submission strategies, coordinate evidence generation, and manage end-to-end communications with EU Notified Bodies, EMA, and the FDA, including FDA PMA/Q-submissions and IVDR Annex XIV applications.
-
What challenges exist when running combined studies under both CTR and IVDR?
Combined studies face regulatory misalignment — IVDR and CTR have different requirements, timelines, and submission pathways. MDx helps sponsors navigate these complexities by developing integrated strategies, ensuring both clinical and performance data meet the distinct expectations of medical device and medicinal product regulators.

Let’s Accelerate Your CDx to Market
Whether you’re co-developing a CDx with a pharma partner or preparing your first IVDR Annex XIV submission, MDx is your full-service companion diagnostics CRO for regulatory success and global approval, delivering ISO 20916 studies, EU/UK legal representative, and FDA PMA/Q-sub support.
Your CDx. Our Expertise. Global Impact.

