Precision medicine needs alignment, not lighter rules
Europe’s precision medicine ecosystem advances when diagnostics and medicines move in lockstep. The real bottleneck is not regulation itself; it is the structural divergence between the Clinical Trials Regulation and the IVDR. In our work across oncology and rare disease programmes, we repeatedly see how separate clocks, committees, documentation and reporting streams create avoidable friction. Our message at MPP 2025 was simple: bring both tracks together from the start and design one plan that satisfies clinical utility and regulatory rigor at the same time.
CDx and drug trials: bridging IVDR and CTR
When a biomarker guides enrolment or treatment and the assay is not CE-marked for that purpose, Article 58 triggers a performance study alongside the medicinal trial. That study’s endpoints, analytical validation and risk–benefit narrative must be coherent with the drug protocol, because outcomes like PFS, OS and ORR depend on the test defining the right population. Misalignment costs time; EFPIA’s 2023 evidence shows many sponsors facing six to twelve-month delays linked to IVDR requirements, which ultimately pushes patient access further out.
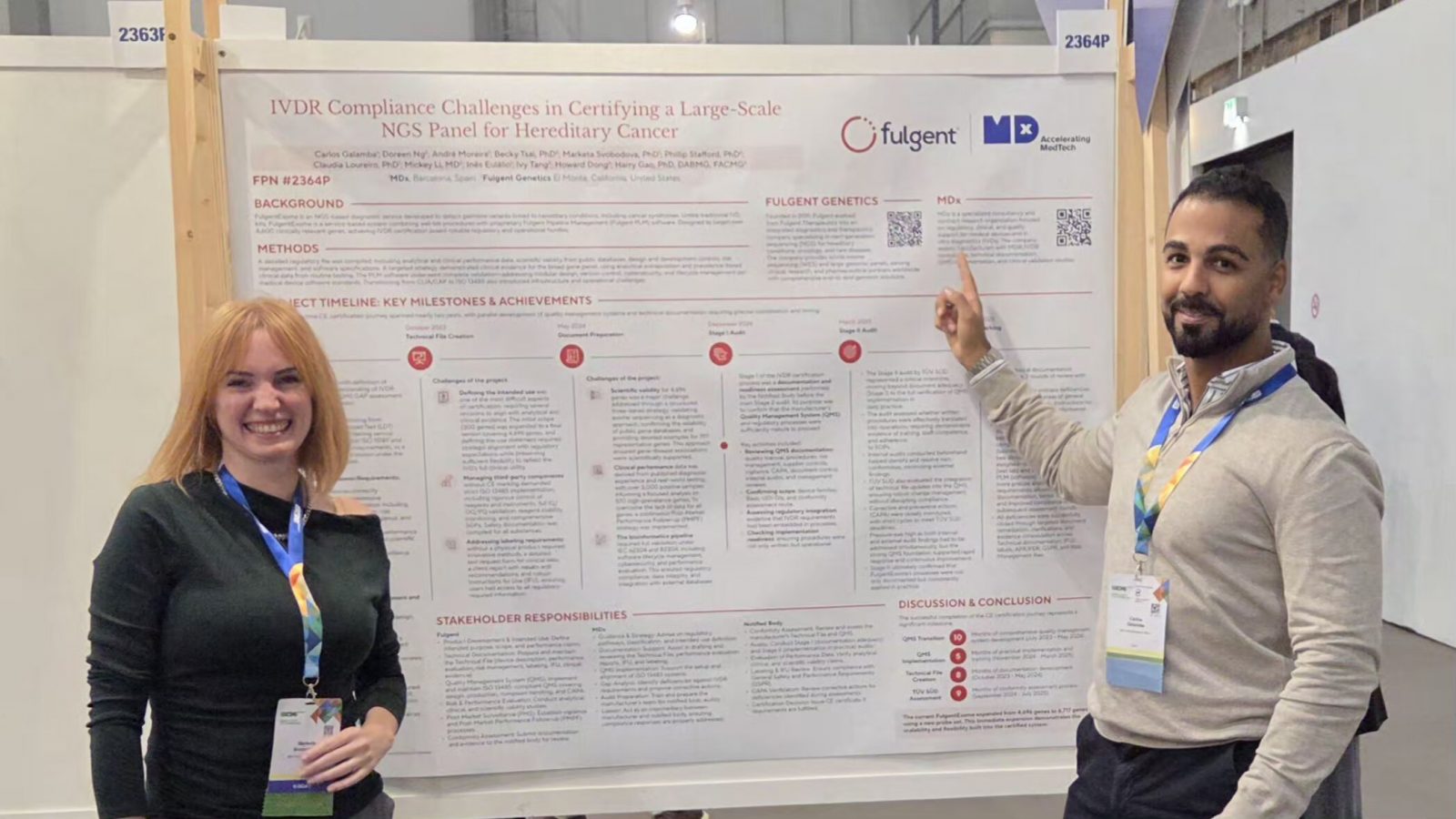
What MDx CRO presented at MPP 2025
At the Medtech & Pharma Platform Annual Conference on 16 September 2025 at the Novartis Campus in Basel, Carlos Galamba, CEO at MDx CRO, joined the session “IVDs, CDx & Personalized Medicine: Moving from Compliance to Innovation,” chaired by Fatima Bennai-Sanfourche of Bayer and Andreas Emmendoerffer of Roche. The panel brought together Antonella Baron from the European Medicines Agency, Heike Möhlig-Zuttermeister of TÜV SÜD, and patient advocate David Haerry of Positive Council Switzerland, alongside MDx.

Evidence on delays and the impact on patients
The data are unambiguous: EFPIA’s survey indicates 43 percent of companies estimated six to twelve-month delays due to IVDR, with downstream consequences for trial starts and patient inclusion across major disease areas. Every month lost to mis-sequenced processes or unclear governance is a month patients wait for targeted therapies.
Signals from regulators, notified bodies, and patient advocates
The discussion reflected a clear appetite for convergence. EMA perspectives on embedding companion diagnostics under IVDR, TÜV SÜD’s insights on conformity assessment for CDx, and the patient community’s call for earlier access all point in the same direction: coordinated planning and earlier dialogue across agencies, notified bodies and sponsors.
Key insights from the MPP panel and fellow presenters
Compliance versus innovation is the wrong debate. The practical path forward is compliance and innovation together: a single evidence plan, shared endpoints, and a unified risk–benefit narrative that treats the diagnostic and the drug as interdependent elements of one therapy journey. That is how companion diagnostics under IVDR accelerate, rather than delay, precision medicine.
How MDx CRO accelerates CDx from design to approval
MDx builds one cross-functional plan from day one, aligning clinical and device protocols, mapping Article 58 triggers, and sequencing submissions so site start-up and “first sample tested” are not held back by documentation gaps. Our teams scrutinise analytical validation, prepare CPSPs and Annex XIV packages aligned to ISO 20916, and train investigators on device-specific safety reporting and sample flows across multilingual EU sites. This integrated approach has delivered a consistent approval track record for CDx submissions.
Operational playbook for combined studies in Europe
Effective combined studies require clear governance between drug and device sponsors, modular and wave submissions across Member States, separate informed consents for the CDx component where appropriate, and proactive scientific advice with EMA or NCAs for borderline cases. With local regulatory intelligence and language capability across Europe, we coordinate roles and documentation so CTR and IVDR remain synchronised throughout the study lifecycle.
Acknowledgment to MPP and our co-presenters
Our thanks to the Medtech & Pharma Platform Association for convening this timely discussion and to the session chairs and speakers who brought regulatory, conformity assessment and patient perspectives to the same table: Fatima Bennai-Sanfourche, Andreas Emmendoerffer, Antonella Baron, Heike Möhlig-Zuttermeister and David Haerry.
Partner with MDx CRO to make CDx work under IVDR
If your programme depends on biomarker-driven enrolment or treatment decisions, partner with a team that speaks CTR and IVDR fluently. MDx CRO compresses timelines, de-risks submissions and delivers companion diagnostics that make precision medicine real for the patients who need it most